We are developing Raman spectroscopy as an in situ cancer detection and characterization technique. Skin cancers are our current focus as we study live cells and whole tissue specimens. Our published Raman study of live metastatic melanoma versus normal skin fibroblast cells employed principal component analysis and linear discriminant analysis to reveal significantly higher RNA levels in the nucleoli of normal fibroblasts and higher amounts of lipid and collagen in the cytoplasm of the melanoma cells. We are also working on developing a fiber-optic-based mobile Raman system for use in skin cancer clinics, as well as combining laser ablation as a method for removing cancers and RS as a cancer detection and guidance system for the ablation laser. Our publication in Lasers and Surgery and Medicine demonstrated the feasibility of this approach.
AC Terentis, SA Fox, SJ Friedman, ES Spencer, Confocal Raman microspectroscopy discriminates live human metastatic melanoma and skin fibroblast cells, Journal of Raman Spectroscopy, 2013:44(9);1205-1216.
SA Fox, A Shanblatt, H Beckman, J Strasswimmer, AC Terentis, Raman Spectroscopy Differentiates Squamous Cell Carcinoma (SCC) From Normal Skin Following Treatment with a High-Powered CO2 Laser, Lasers in Surgery and Medicine, 2014:46(10);757–772. [PMID: 25345858]
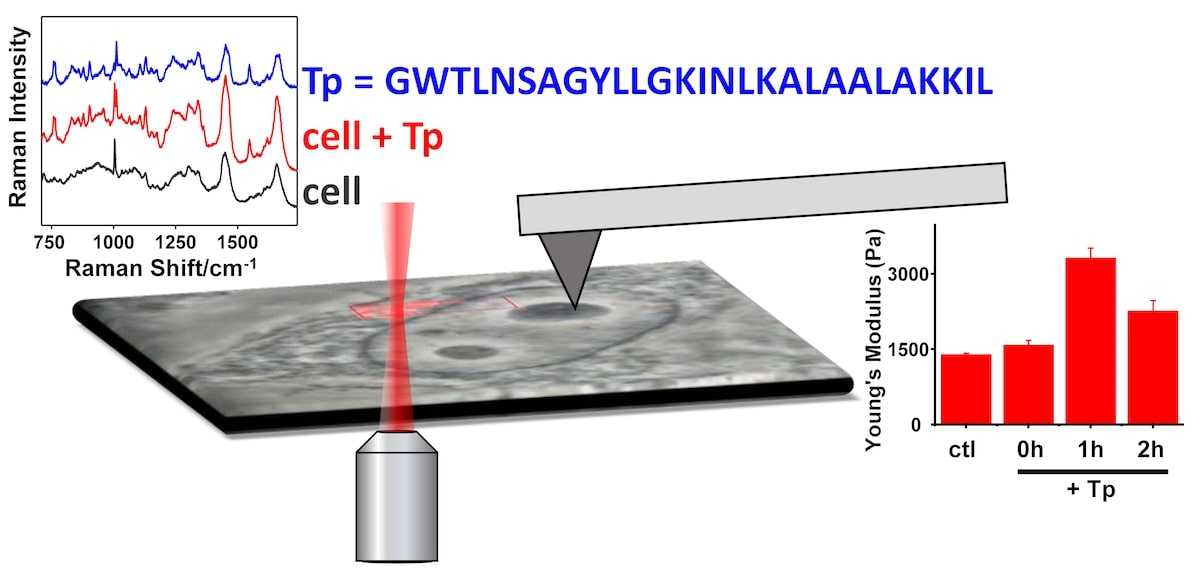
We are studying the mechanisms by which cell penetrating peptides (CPPs) penetrate cells. CPPs are of great interest for their potential use in drug delivery, but the structural determinants of CPP function are not well understood. Currently our focus is on CPP secondary structure-function relationships. We developed a novel method for detecting CPPs within single, living cells using Raman micro-spectroscopy. With this method, we were able to measure Raman spectra of penetratin inside melanoma cells and to determine that its secondary structure was mainly beta-sheet. Having established this new method for the study of CPPs in cells, our research has continued to look at other CPPs. Our more recent work on transportan employed both Raman micro-spectroscopy to monitor the time-dependent distribution of the peptide within living cells, and atomic force microscopy to determine that transportan increased the stiffness of metastatic melanoma cells.
PJ Cosme, J Ye, S Sears, EP Wojcikiewicz, AC Terentis, Label-Free Confocal Raman Mapping of Transportan in Melanoma Cells, Molecular Pharmaceutics, 2018:15(3);851-860. [PMID: 29397737]
AC Terentis, J Ye, Peptide detection and structure determination in live cells using confocal Raman microscopy. In Peptide Modifications to Increase Metabolic Stability and Activity; P Cudic, Ed. Humana Press: New York, 2013; Vol. 1081, pp 211-236. From Methods in Molecular Biology series. [PMID: 24014442]
J Ye, SA Fox, M Cudic, EM Rezler, JL Lauer, GB Fields, AC Terentis, Determination of Penetratin Secondary Structure in Live Cells with Raman Microscopy, Journal of the American Chemical Society, 2010:132(3);980-988. [PMID: 20041639]
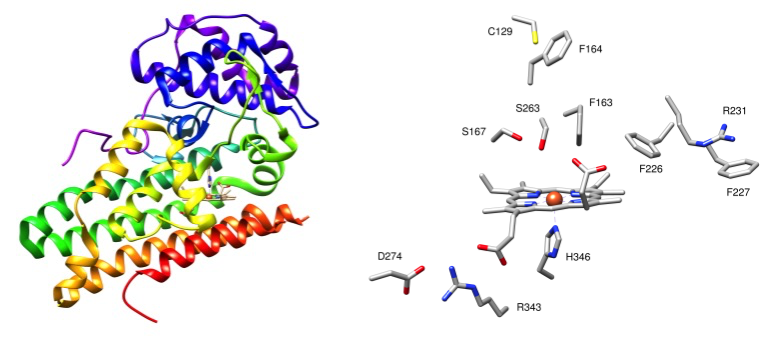
Our research has focused primarily in cancer-related, amino-acid metabolizing enzymes such as Indoleamine 2, 3-dioxygenase (IDO) and methionine gamma lyase (MGL). In collaboration with Dr. Shane Thomas's group at the University of New South Wales, we study novel physiological functions and modes of inhibition of IDO using a variety of techniques such as resonance Raman spectroscopy, UV-Vis binding studies, enzyme kinetics, and circular dichroism spectroscopy. In collaboration with Dr. Venkatachalam at Nova Southeastern University, and using a similar array of techniques, we are elucidating the catalytic mechanism of MGL activity. To complement our experimental studies, we perform QM calculations to determine the structures and energies of reaction intermediates and transition states. Over the years, we have also performed resonance Raman studies of other enzymes and proteins for a variety of other collaborative research groups.
YJ Lim, TC Foo, AWS Yeung, X Tu, Y Ma, CL Hawkins, PK Witting, GN Jameson, AC Terentis, SR Thomas, Human Indoleamine 2,3-Dioxygenase 1 is a Novel Mammalian Nitrite Reductase, Biochemistry, 2019:58(7):974-986. [PMID: 30585477]
CJ Corcoran, CC Tang, V Lykourinou, AC Terentis, A Andgerhofer, L-J Ming, To be structurally well-defined or not to be, that is not the question for iron(III)–poly(4-Vinylpyridine-co-acrylamide) to exhibit catechol dioxygenase activity!, Catalysis Communications, 2018:106;87-91. [https://doi.org/10.1016/j.catcom.2017.11.006]
TC Foo, AC Terentis, KV Venkatachalam, A continuous spectrophotometric assay and nonlinear kinetic analysis of methionine γ-lyase catalysis, Analytical Biochemistry, 2016:507;21-26. [PMID: 27235171]
AWS Yeung, AC Terentis, NJC King, SR Thomas, Role of Indoleamine 2,3-Dioxygenase in Health and Disease, Clinical Science, 2015:129(7);601-672. [PMID: 26186743]
M Freewan, MD Rees, TS Sempértegui Plaza, E Glaros, YJ Lim, X-S Wang, AWS Yeung, PK Witting, AC Terentis, SR Thomas, Human Indoleamine 2,3-Dioxygenase is a Catalyst of Physiological Heme Peroxidase Reactions – Implications for the Inhibition of Dioxygenase Activity by Hydrogen Peroxide, Journal of Biological Chemistry, 2013:288(3);1548-1567. [PMID: 23209301]
AC Terentis, M Freewan, TS Sempértegui Plaza, M Raftery, R Stocker, SR Thomas, The Selenazal Drug Ebselen Potently Inhibits Indoleamine 2,3-Dioxygenase by Targeting Enzyme Cysteine Residues, Biochemistry, 2010:49(3);591-600. [PMID: 20000778]
SW Vetter, AC Terentis, RL Osborne, JH Dawson, DB Goodin, Replacement of the Axial Histidine Heme Ligand with Cysteine in Nitrophorin-1: Spectroscopic and Crystallographic Characterization, Journal of Biological Inorganic Chemistry, 2009:14(2);179-191. [PMID: 18923851]
SR Thomas, AC Terentis, H Cai, O Takikawa, A Levina, PA Lay, M Freewan, R Stocker, Post-translational regulation of human indolamine 2,3-dioxygenase activity by nitric oxide, Journal of Biological Chemistry, 2007:282(33);23778-23787. [PMID: 17535808]
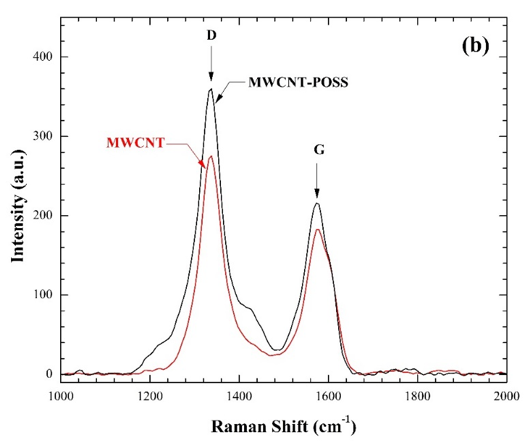
In collaboration with Dr. Hassan Mahfuz in the College of Engineering at FAU, we study the structural and mechanical properties of novel polymer composite materials impregnated with carbon nanomaterials, which are being developed for improved performance in marine and aeronautical applications.
C Gapstur, H Mahfuz, J Hashemi, AC Terentis, Enhancing Fracture Toughness and Stress Energy Release Rate of Vinyl Ester Matrix Using Dual Reinforcement of CNT and GNP, MRS Advances, 2018:3(15-16);867-873.
SM Sabet, H Mahfuz, AC Terentis, M Nezakat, J Hashemi, Effects of POSS Functionalization of Carbon Nanotubes on Microstructure and Thermo-Mechanical Behavior of Carbon Nanotube/Polymer Nanocomposites, Journal of Materials Science, 2018:53(12);8963-8977.
SM Sabet, H Mahfuz, AC Terentis, J Hashemi, B Boesl, A facile approach to the synthesis of multi-walled carbon nanotube-polyhedral oligomeric silsesquioxane (POSS) nanohybrids, Materials Letters, 2016:168;9-12.
AM Knapinska, D Tokmina-Roszyk, S Amar, M Tokmina-Roszyk, VN Mochalin, Y Gogotsi, P Cosme, AC Terentis, GB Fields, Solid-Phase Synthesis, Characterization, and Cellular Activities of Collagen-Model Nanodiamond-Peptide Conjugates, Peptide Science, 2015:104(3);186-195. [PMID: 25753561]
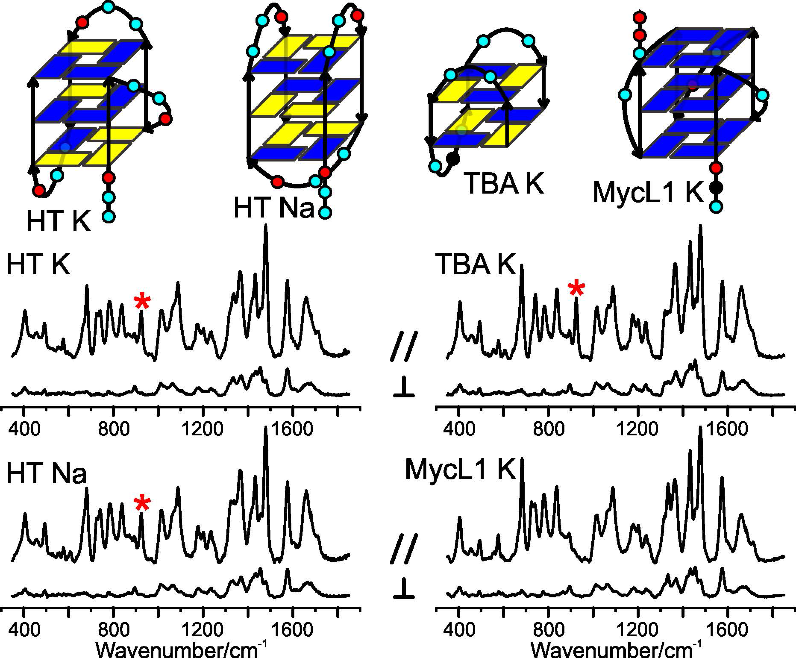
Some oligonucleotides that are rich in guanine bases can fold into interesting structures called G-quadruplexes (G4s). There is evidence that G4s play a role in regulating gene expression, which may present targets for anti-cancer therapies. Our interest is in using Raman spectroscopy to characterize the rich topological landscape of G4s, which in turn may aid the development of small molecules that selectively target G4s.
SJ Friedman, AC Terentis, Analysis of G-Quadruplex Conformations using Raman and Polarized Raman Spectroscopy, Journal of Raman Spectroscopy, 2016:47(3);259-268. [DOI: 10.1002/jrs.4823]