ALZHEIMER'S DISEASE

The amyloid-β precursor protein (APP) undergoes proteolysis by β- and γ-secretases to form amyloid-β peptides (Aβ), which is a hallmark of Alzheimer's disease (AD). Recent findings suggest a possible role of O-glycosylation on APP's proteolytic processing and subsequent fate for AD-related pathology. To date, stimulating and inhibiting effects of glycosylation on enzyme activity have been reported. Thus, a better understanding of the role of O-glycosylation on the balance between production and clearance of Aβ peptides is necessary to decipher the role of O-glycosylation in the initiating events of AD pathogenic processes.

|
|
Tau is a microtubule-associated protein essential for maintaining the stability and function of microtubules in neurons. In Alzheimer’s disease (AD) and other tauopathies, tau undergoes abnormal hyperphosphorylation, leading to its detachment from microtubules and subsequent aggregation into paired helical filaments. These filaments accumulate to form neurofibrillary tangles (NFTs)-intracellular aggregates of hyperphosphorylated tau that are a hallmark biomarker of AD. In a healthy brain, tau is typically modified by O-linked β-N-acetylglucosamine (O-GlcNAc) and contains only a limited number of phosphorylation sites. We hypothesize that a competitive relationship between O-GlcNAcylation and phosphorylation may regulate tau aggregation, presenting a potential avenue for therapeutic intervention. The primary objective of our group is to chemically synthesize tau protein models with defined glycosylation and phosphorylation patterns. These models will allow us to investigate how specific post-translational modifications influence tau’s structure and aggregation behavior. A deeper understanding of these mechanisms could pave the way for innovative therapeutic strategies design to prevent or slow down the progression of Alzheimer’s and Parkinson’s diseases.

FUNDING
This project is funded by the Florida Department of Health: Ed and Ethel Moore Alzheimer’s Disease Research Program.
CANCER

MUC1, a high-molecular weight glycoprotein, secreted and expressed at the cellular surface of various epithelial tissues. The structure of MUC1 consists of the intracellular C-terminal domain (MUC1-C) and a large extracellular N-terminal tandem repeat domain (MUC1-N), which are non-covalently linked to a smaller subunit containing an autocatalytic cleavage SEA-module and the transmembrane domain (TM).
In normal cells, MUC1-N is heavily glycosylated and extends from the cell surface up to 500 nm. MUC1-N is covered with branched core 2 O-glycans [Galb1-3(GlcNAcb1-6)GalNAc] with lactosamine extensions. This heavy glycosylation plays a crucial role in forming a protective barrier on epithelial cell surfaces, preventing damage and infection.
In cancer, tumor-associated MUC1 (TA MUC1) becomes overexpressed, hypoglycosylated and redistributed over the entire surface of the cell due to a loss in apical-basal polarity. The long-branched glycan chains are found to be truncated and/or capped with sialic acid. The most common tumor associated carbohydrate antigens (TACAs) formed from incomplete synthesis are GalNAcα-O-Ser/Thr (Tn, Thomsen Nouveau, CD175), Neu5Acα2,6-GalNAcα-O-Ser/Thr (sTn, sialyl Tn, CD175s), Galβ1,3-GalNAcα-O-Ser/Thr (TF, Thomsen-Friedenreich, CD176, T antigen) and Neu5Acα2,6- and Neu5Acα2,3-Galβ1,3-GalNAcα-O-Ser/Thr (2,6-sTF, 2,3-sTF). Their expression levels have been used as biomarkers of poor prognosis.
Cell-surface TACA-lectin interactions: Role in tumor progression, metastasis and immune evasion
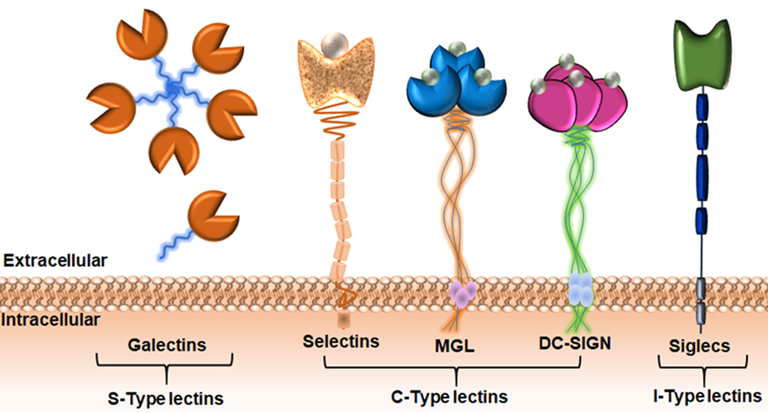
Carbohydrate-binding proteins, lectins, are the main binding partners of TACAs on the surface of the cell. These interactions often lead to the creation of a pro-tumor microenvironment, favoring tumor progression and metastasis. In addition, glycan-protein recognition may contribute to tumor progression through evasion of antitumor immune-responses. It has recently been proposed that the tumor glyco-code may be considered as novel immune checkpoints.

|
|
Cell-surface TACAs: Targets for cancer immunotherapy
TACAs of MUC1 are antigenic due to the lack of their expression on healthy cells, making them ideal targets for anti-tumor immune prevention and therapy. Indeed, a broad spectrum of natural anti-TACA antibodies are present in the human serum. These anti-glycan antibodies are IgM type, however, recently the IgG type cancer-associated autoantibodies have been detected toward distinct O-glycopeptide epitopes. The presence of circulating naturally occurring anti-MUC1 antibodies has been correlated with better disease prognosis in cancer patients.

OUR APPROACH TO STUDY MUC1 GLYCOBIOLOGY

The functional significance on the molecular level of the changes in O-linked MUC1 glycosylation in cancer is still difficult to determine due to the intrinsic genetic instability and cellular heterogeneity of tumors. In addition, the cluster glycoside effect and steric hindrance effect of neighboring glycans on binding to glycan-binding proteins (lectins) have not been well characterized.
We are taking advantage of synthetic chemistry strategies developed in our group to control the MUC1 epitope complexity by designing the glycopeptide models with an increasing degree of multivalency (individual glycopeptides with varying number of glycan attachments, glycopeptide libraries offering access to multiple combinations of structures and presentations of glycans relevant to natural glycan arrays found on cancer cells, and branched multivalent construct) to systematically elucidate structure-binding relationships.
Consequently, these synthetic model compounds with the well-defined diversity of the glycan ligand-based chemical space are used to study the glycoside cluster and steric hindrance effects of neighboring glycans on binding to lectins. ITC and AFM measurements are used in the binding studies to establish in-depth thermodynamic and kinetic binding profiles of MUC1-lectin interactions.
We are also developing a novel analytical assay for low affinity glycan-protein interactions based on AlphaScreen technology.
These tools play a crucial role in deepening our understanding of MUC1's impact on cancer cell biology and immunology. By leveraging them, we can gain valuable insights into MUC1's function, its interactions within the tumor microenvironment, and its potential as a therapeutic target. Based on our experimental evidence of importance of the peptide part in the recognition process, a detailed elucidation of the affinity and specificity amongst lectins for glycans as they exist in their natural cellular context, conjugated to a protein, is needed for biomedical applications.
FUNDING
This project is funded by the National Institute of Health/National Cancer Institute (R21 and R15 grants).